In recent years, the herpes simplex virus 1 (HSV1) has emerged as one of the most advanced and successful oncolytic viruses. But despite having received approval from the US Food and Drug Administration (FDA) in 2015 for use in melanomas, certain limitations – such as sustained viral replication within tumours – still restrict its efficacy as a successful anti-cancer therapy.
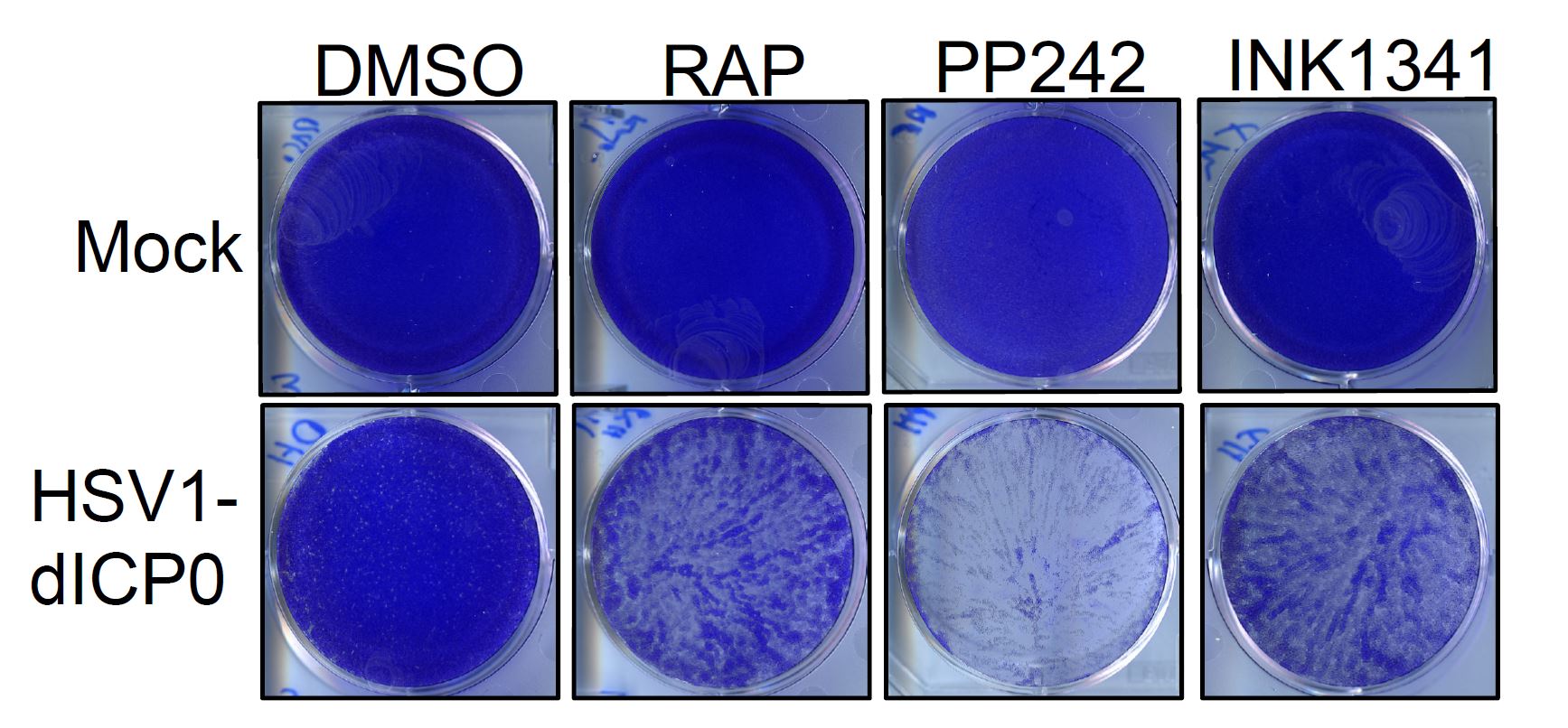
In this context, a new study published by a team of TFRI-funded researchers based in Ottawa and Montreal could mark the beginning of a new era for the use of HSV1 as a therapeutic option for cancer. The study, published in PLOS Pathogens (August 2018), found that combining HSV1 with mTOR inhibitors (which suppress the Mammalian Target of Rapamycin (mTOR), an important enzyme found to be hyperactive in cancer cells) improves viral replication within tumour cells with dysregulated protein synthesis, boosting the virus’ efficacy.
“What this study revealed is that adding active-site mTOR inhibitors to cancer cells with dysregulated protein synthesis resulted in an altered cellular state that preferentially benefits the replication of HSV1,” explains Dr. Tommy Alain (University of Ottawa and Children’s Hospital of Eastern Ontario), a viral oncologist and the paper’s senior author. “Such combination could therefore be developed to improve both mTOR- and HSV1-targeted anti-cancer therapies.”
To reach this conclusion, the team combined active site mTOR inhibitors (asTORi) with a specific variation of HSV1 (HSV1-dICP0) in cancer cells and a mouse mammary cancer xenograft. The combination therapy reduced tumour size of this aggressive syngeneic breast cancer and revealed that cancer cells harboring altered mRNA translation via dysregulated eIF4E/4E-BPs axis can be targeted by the combination of asTORi and HSV1-dICP0.
According to Dr. Alain, this occurred because the combination therapy promoted viral replication within certain cancer cells, while actively suppressing the virus in normal cells, making the combination therapy a more effective way to specifically target the cancerous cells.
“Clinicians or oncologists treating patients with either mTOR inhibitors or an oncolytic HSV1 may consider combining the two therapies for improved therapeutic responses, especially if the cancer displays signs of dysregulated protein synthesis,” says Dr. Alain.
Study
Active-site mTOR inhibitors augment HSV1-dICP0 infection in cancer cells via dysregulated eIF4E/4E-BP axis
Authors
Chadi Zakaria, Polen Sean, Huy-Dung Hoang, Louis-Phillipe Leroux, Margaret Watson, Samuel Tekeste Workenhe, Jaclyn Hearnden, Dana Pearl, Vinh Tai Truong, Nathaniel Robichaud, Akiko Yanagiya, Soroush Tahmasebi, Seyed Mehdi Jafarnejad, Jian-Jun Jia, Adrian Pelin, Jean-Simon Diallo, Fabrice Le Boeuf, John Cameron Bell, Karen Louise Mossman, Tyson Ernst Graber, Maritza Jaramillo, Nahum Sonenberg, Tommy Alain
Funding
This work was supported by a The Terry Fox New Frontiers Program Project Grant: Canadian Oncolytic Virus Consortium (COVCo).