Despite decades of advancement in treatment, cancer spreading to multiple parts of the body remains one of the biggest challenges facing patients and their health-care teams.
This is particularly true for osteosarcoma, the most common bone cancer in children and teenagers, and the disease that claimed Terry Fox’s life. While survival rates are approximately 70 per cent for people with localized disease, there is a high risk of metastatic spread to the lungs, after which the odds of survival fall dramatically to 20 per cent or less.
“That’s what we’re trying to change,” says Dr. Poul Sorensen, a professor at the University of British Columbia and a Terry Fox-funded researcher, whose team has identified a potential drug that could halt the spread of osteosarcoma.
“Our recent findings build on earlier work that was funded by the Terry Fox Research Institute,” says Dr. Michael Lizardo, first author of the recent study and scientist in the Sorensen lab.
In the study, the team demonstrated that this compound can reduce lung metastasis in mice by more than 90 per cent, while also shrinking the primary tumour.
“This discovery brings much-needed hope to children and youth battling osteosarcoma,” said Dr. Sorensen. “We know how devastating this disease can be if it spreads to the lung, and we have long needed effective tools to prevent and treat that. With this drug we might be able to stop lung metastasis and give young people a much better chance at beating this disease.”
While this research focused on children and youth with osteosarcoma, the team hopes to continue building on this work to help patients with other cancers as well.
“What we learn in these pediatric or less-common (or rare) tumours often has direct applicability to adult malignancies and other tumour types,” says Dr. Sorensen.
Weakening cancer cells
In this study, the drug, known as an eIF4A1 inhibitor, blocks the ability of osteosarcoma cells to protect themselves and survive in the lung’s harsh microenvironment.
The lungs are known to be an inhospitable environment for foreign cancer cells, with the majority of cancer cells being killed by nutrient deprivation and exposure to high levels of oxidative stress. However, in some cases, a small number of cells are able to adapt to such conditions and seed the growth of a metastatic tumour.
“It really only takes a small population of osteosarcoma cells to adapt, survive and proliferate in the lung,” said Dr. Lizardo, a scientist in the Sorensen lab. “This drug essentially strips those cells of their protective armour so that they’re more susceptible to oxidative stress and unable to take root in the lung.”
As part of the study, the researchers showed that some osteosarcoma cells are able to survive within the lung by producing a protein, NRF2, which acts as a protective antioxidant. They identified the pathway by which the protein is produced and collaborated with the late Dr. Jerry Pelletier of McGill University, who pioneered the development of an eIF4A1 inhibitor compound (CR-1-31B) that disrupts that process.
The UBC team tested the CR-1-31B compound in mice in collaboration with Dr. Alejandro Sweet-Cordero from the University of California San Francisco, who provided cell lines of metastatic osteosarcoma from his lab.
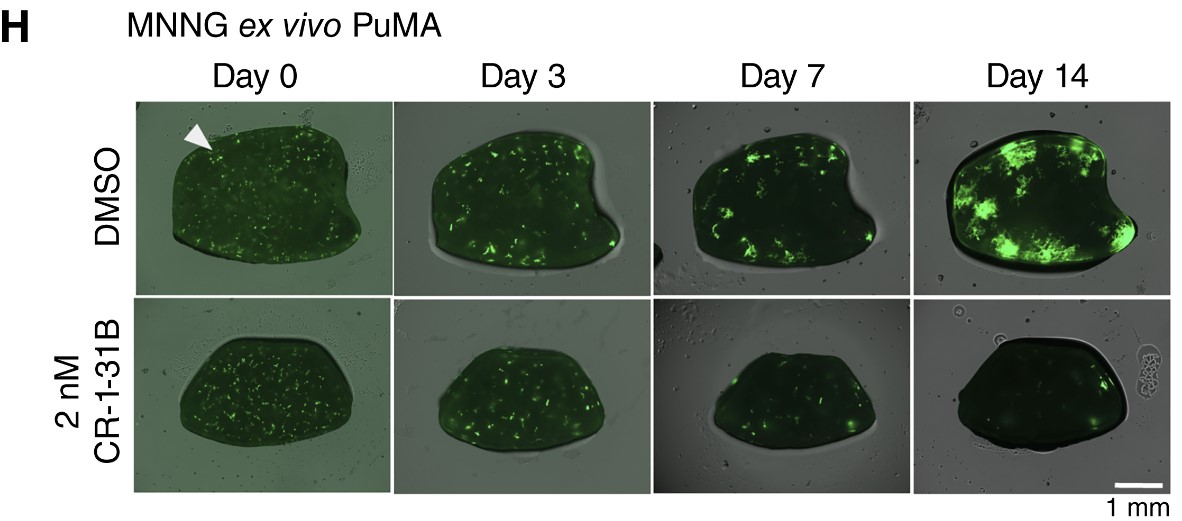
Tumour growth (green) was notably less in mouse lung tissue explants treated with the drug (bottom) compared to untreated mouse lung tissue explants (top).
Sights set on clinical trials
Notably, a second eIF4A1 inhibitor drug, called Zotatifin, also proved successful in halting lung metastasis and has already shown promise in early clinical trials for other conditions. Dr. Sorensen says this could accelerate its potential for human trials in osteosarcoma patients.
“It’s particularly exciting that there is an existing clinical-grade compound. That opens up opportunities to rapidly begin testing this in human trials and could accelerate timelines for a new treatment to reach patients,” he said.
Dr. Sorensen and his team are now in conversations with clinical researchers to bring the promising discovery into human trials. If successful, it could represent a major breakthrough for children and families affected by osteosarcoma, while Dr. Sorensen also notes that there could be implications for treating a range of other cancers that exhibit similar characteristics, such as some forms of lung, breast and pancreatic cancer.
These findings build on research funded by a Terry Fox New Frontiers Program Project Grant: Genomics of forme fruste tumours: new vistas on cancer biology and treatment.
The study was also supported by the BC Cancer Foundation, Alex’s Lemonade Stand Foundation and the Canadian Institutes of Health Research.