The discovery of a new pathway for the treatment of castrate-resistant prostate cancer has scientists in Montreal excited about how repurposed and new drugs targeting it may benefit patients.
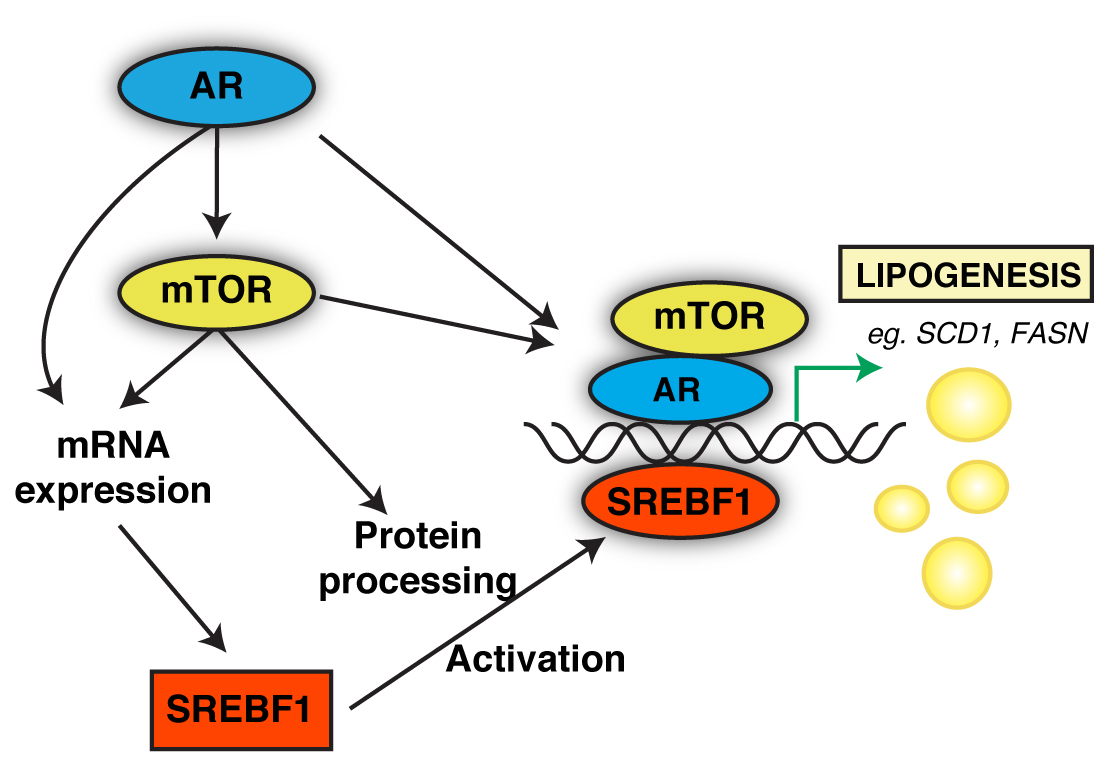
In a study published in Molecular Cancer Research (September 2018), the TFRI-funded team led by Dr. Vincent Giguère (Goodman Cancer Research Centre, McGill University) identified a newly discovered downstream effector of the androgen receptor (AR)/mammalian target of rapamycin (mTOR)axis, a pathway known as SREBF1 that was found to be directly responsible for controlling prostate cancer cell metabolism.
“We found that SREBF1 expression is contingent on the dual activation of the AR/mTOR signaling pathways and is essential in controlling two key metabolic processes in prostate cancer: the balance between usage of citrate for mitochondrial ATP synthesis and de novo lipid synthesis,” explains Dr. Etienne Audet-Walsh, the lead author of the study.
These two metabolic processes allow prostate cancer cells to gain the energy they need to grow and spread, which is why identifying a pathway that controls them is so important: if it can be inhibited genetically or pharmacologically, then perhaps the cancer cells can be starved to death. Sterol regulatory element-binding transcription factor 1 (SREBF1) is in fact targetable, making this discovery even more exciting.
The finding builds on previous groundbreaking work by the team studying cancer metabolism. In 2017, they revealed how the nuclear fraction of mTOR and androgen receptors worked together to facilitate the growth and proliferation of prostate cancer cells. The team has continued to dive deeper into the relationship between mTOR and AR to gain a greater understanding of the metabolic processes involved in tumour proliferation and potential novel therapeutics.
“Our study has found that pharmacologic inhibition of SREBF1 impairs prostate cancer cell proliferation in vitro and in vivo, and what’s best is that this regulatory pathway can be targeted by several pharmacological approaches already in use to treat other diseases,” says Dr. Giguère. “Our work supports drug-repurposing for the treatment of prostate cancer and thus will potentially lead to the approval of new medications for prostate cancer following appearance of resistance.”
Study
SREBF1 Activity Is Regulated by an AR/mTOR Nuclear Axis in Prostate Cancer
Authors
Etienne Audet-Walsh, Mathieu Vernier, Tracey Yee, Chloé Laflamme, Susan Li, Yonghong Chen, and Vincent Giguère
Funding
This work was partially supported by a grant to the Terry Fox Program Program Grant in Oncometabolism and the Molecular Pathways that Fuel Cancer