A Toronto-based group of TFRI-funded scientists has discovered a new therapeutic target that could bring hope to patients with triple-negative breast cancer (TNBC), an aggressive breast cancer with no effective treatments.
Led by Dr. Eldad Zacksenhaus (Toronto General Research Institute, UofT), the research team used mouse models and human cell lines to help identify CDC25, a phosphate enzyme that promotes cell proliferation, as a common therapeutic target for diverse TNBCs, including tumours with mutations in RB1, PTEN and p53, three tumour-suppression genes frequently inactivated in TNBC.
The team found that inhibiting CDC25 pharmacologically or genetically promoted cancer cell death in tumours that were RB1-deficient. This breakthrough, which was published in Cell Reports (April 2018), marks a big leap forward in this area of cancer research. Until now, researchers have struggled to find ways to treat RB1-deficient tumours, as they are unresponsive to CDK4/6 inhibitors, which are commonly used against cancer cells expressing the RB1 gene.
Researchers are hoping that this discovery will inspire others in the scientific community to develop new CDC25 inhibitors that work alone or in combination with other drugs to bring much needed hope to people living with TNBC.
“Although many such inhibitors are available; no anti-CDC25 drug has been approved for use in the clinic yet,” said Dr. Zacksenhaus. “Hopefully these findings will renew interest in the development of safe and effective CDC25 inhibitors that can be used in clinics.”
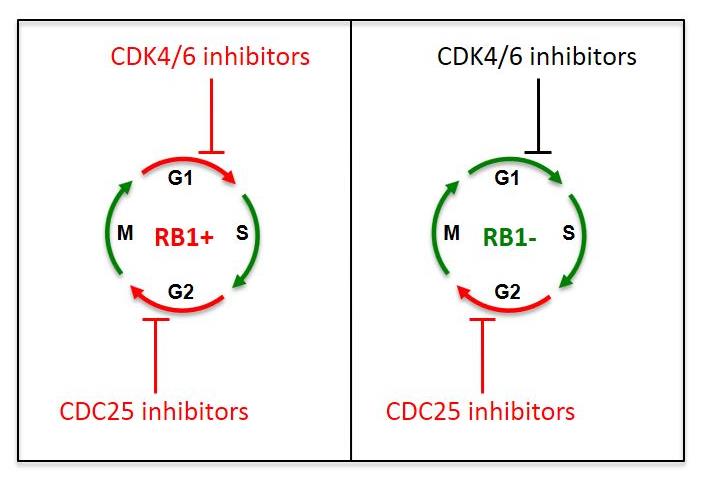
The diagram on the left shows how RB1-positive TNBC cells are sensitive to both CDK4/6 inhibitors and CDC25 inhibitors, while the diagram on the right shows how RB1-deficient TNBC cells are resistant to CDK4/6 inhibitors but remain sensitive to CDC25 inhibition.
Study
Identification of CDC25 as a Common Therapeutic Target for Triple-Negative Breast Cancer
Authors
Jeff C. Liu, Letizia Granieri, Mariusz Shrestha, Dong-Yu Wang, Ioulia Vorobieva, Elizabeth A. Rubie, Rob Jones, YoungJun Ju, Giovanna Pellecchia, Zhe Jiang, Carlo A. Palmerini, Yaacov Ben-David, Sean E. Egan, James R. Woodgett, Gary D. Bader, Alessandro Datti, and Eldad Zacksenhaus
Funding
This study was partially funded by a Terry Fox New Frontiers Program Project Grant for Killing the Hydra: Genetic dissection of actionable targets required for maintenance of metastatic disease